8th DOH Steering Committee Meeting for the Clinical Trials on COVID-19
- admin
- November 5, 2020
- 1:56 pm
Undersecrertary Maria Rosario Vergeire of the Health Regulation Team presided the 8th meeting via Zoom. Once again, the latest status of the WHO Solidarity trials for COVID-19 treatments and vaccines were presented. These include updates on the treatment arms for the Solidarity Trial for COVID-19 investigational drugs, wherein the Interferon arm was totally discontinued, while another treatment arm – Acalabrutinib, an anti-cancer drug, is being considered for inclusion in the study. Acalabrutinib is expected to be released anytime soon upon clearance by the concerned offices within the Bureau of Customs. Updates on the preparation for the WHO Solidarity Vaccine Trial (SVT) were also provided by Dir. Jaime C. Montoya, Executive Director of the DOST – Philippine Council for Health Research and Development (PCHRD), Mr Joh Paolo Tonelete of the WHO Philippines and representatives of other DOH offices who are identified members of the Steering Committee. Among the preparations that are crucial in the SVT are the availability of incidence data of COVID-19 in the Philippines, which will help in identifying clinical trial sites as well as accessibility to ultra-low temperature (ULT) freezers that will house the investigational vaccines. The expected start of the clinical trial for vaccines is in December, 2020.
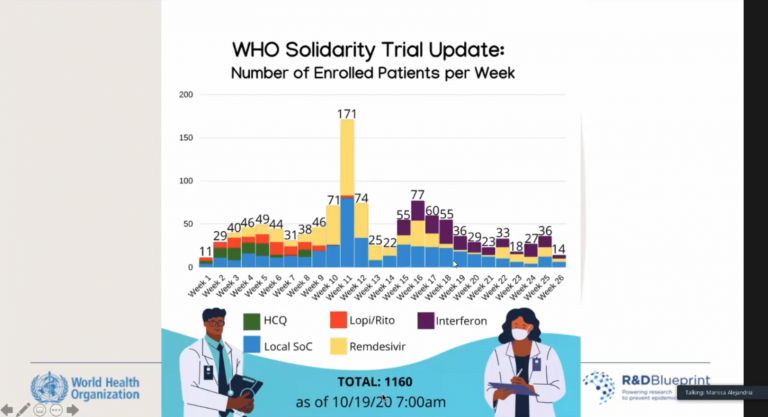
A brief update on the number of enrolled patients per week that was reported by Dr. Marissa Alejandria
On the other hand, the Favipiravir (Avigan) trial, which is subject for protocol amendment, has increased its target patients from 96 to 114. The study team has commenced the recruitment of patients that fit the criteria for inclusion in the clinical trial as they abide with the current protocol. There is still a need for the approval of the ethics review boards and the regulatory agency (FDA Philippines) on the protocol modification before the clinical trial team can proceed with the robust changes in terms of enrolling more patients.
Lastly, the Steering Committee has already selected the members of the Data Safety Monitoring Committee (DSMC) as each representative voted. Once the members are complete, the DOH-HPDPB will issue a Department Personnel Order (DPO) to officially start the engagement of the DSMC in the approved DOH-sponsored clinical trials.
The meeting adjourned early despite discussing thoroughly all the topics in the jampacked agenda. The SC members will again convene after one week.
Related Posts
Contact Us:
Telephone Number: 02-8757734 Loc. 253
Address: 4th Floor, Philippine Blood Center, Lung Center Compound, Quezon Avenue, Quezon City
Email Address: dohncpam@gmail.com


