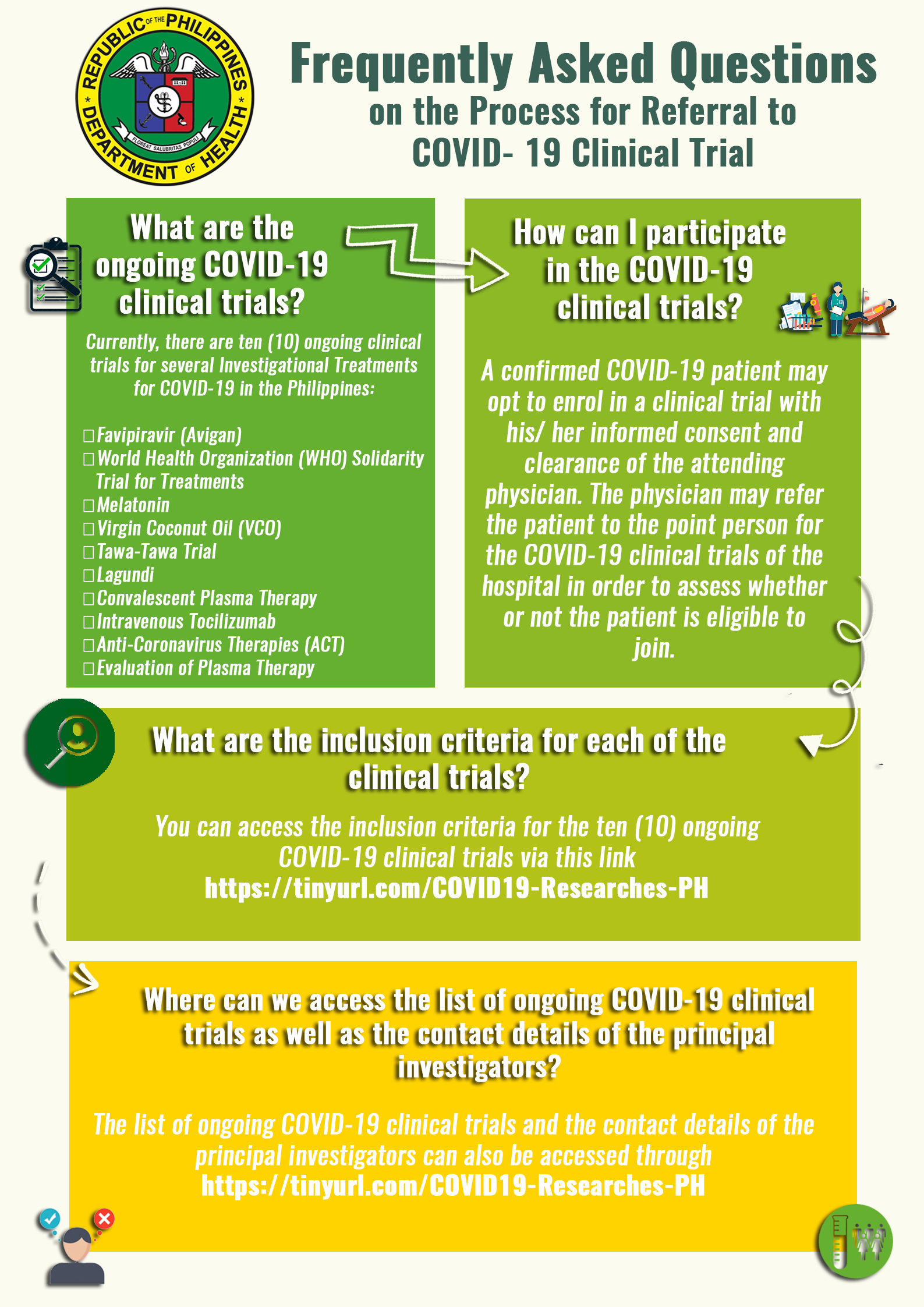
DOH Steering Committee for the Clinical Trials for COVID-19
The National Steering Committee for the conduct of clinical trials for investigational therapies for COVID-19 is a body that was created by virtue of DPO 2020-1377 to ensure the necessary oversight in the screening and administration of the investigational therapies for COVID-19 patients and ensure their safety and well-being.
Related Issuances
News and updates regarding on the DOH Steering Committee for the Clinical Trials for COVID-19

Conduct of the 2022 Antimicrobial Stewardship (AMS) Blended Learning Framework Program for Primary Health Care (PHC) Facilities through Online Platforms
The Department of Health will conduct series of virtual trainings on Antimicrobial Stewardship this August and October 2022.

DOH clarifies exemptions of COVID-19 Vaccines not listed under the latest PNF.
The Department of Health informs all DOH and CHD officials, Philhealth and DOH hospitals and health facilities on the Exemption from Import Duties, Taxes and Other Fees for the Procurement,

DOH calls for a virtual meeting with hospitals re: COVID-19 medicines tracker
The Department of Health will hold a consultative meeting via Webex platform on 08 June 2022, 2:00-4:00 PM to get the comments and feedback of medical centers, hospitals, sanitaria and specialty hospitals on the newly-developed COVID-19 medicines tracker.

Patient Recruitment and Referral for the World Health Organization (WHO) COVID-19 Solidarity Therapeutic Trial Plus
DOH request its NCR hospitals to facilitate patient recruitment for clinical trial.

Updated Suggested Retail Price (SRPs) for Emergency Essential Medicines due to the Coronavirus Disease 2019 (COVID-19) Health Event as of April 22, 2022
DOH adds Molnupiravir (400 mg) in the Updated SRP List

Updated Suggested Retail Price (SRPs) for Emergency Essential Medicines and Medical Devices due to the Coronavirus Disease 2019 (COVID-19) Health Event as of January 19, 2022
DOH adds Molnupiravir in the Updated SRP List
Contact Us:
Telephone Number: 02-8757734 Loc. 253
Address: 4th Floor, Philippine Blood Center, Lung Center Compound, Quezon Avenue, Quezon City
Email Address: dohncpam@gmail.com